Patient Advocacy and the Choroideremia Market: Shaping Future Treatment Approaches
Choroideremia (CHM) is a rare, X-linked genetic disorder that causes progressive vision loss due to degeneration of the choroid and retina in the eye. It primarily affects males, as the disease is inherited in an X-linked recessive pattern, meaning females are typically carriers and rarely show symptoms. Choroideremia leads to a gradual loss of vision, beginning in childhood and progressing through adolescence and adulthood. As the condition advances, patients experience a loss of central vision, leading to blindness by mid-adulthood in severe cases.
This article provides insights into the choroideremia market, its epidemiology, and market forecast through 2032. We will explore the latest trends in treatment development, epidemiological data, and the competitive landscape in the choroideremia market.
Market Insight
Choroideremia is a condition with significant unmet medical needs, as there are currently no FDA-approved therapies to cure or halt its progression. Treatment efforts are primarily focused on gene therapy, with advancements in molecular genetics offering hope for patients. Gene therapies aim to replace or repair the defective CHM gene, which is responsible for producing the REP1 protein required for proper retinal function.
Several gene-based therapies are currently in clinical trials, with promising results. The first approved treatment, if successful, could revolutionize the management of choroideremia, offering the potential to slow or even halt the progression of vision loss. Additionally, ongoing research into retinal implants and stem cell therapy presents future treatment options that may further improve the quality of life for patients with choroideremia.
Key players in the market are focusing on innovative therapies that target the underlying genetic causes of the disease, with CRISPR technology and gene editing tools emerging as powerful approaches for restoring vision or slowing degeneration. The growing emphasis on personalized medicine and genetic therapy is expected to shape the market's trajectory, leading to more tailored treatment approaches for patients.
Request for sample report @ Choroideremia Market
Epidemiology of Choroideremia
Choroideremia is a rare disorder, with an estimated prevalence of 1 in 50,000 males globally. The disease affects both adults and children, though the symptoms typically manifest in childhood. As the condition is X-linked, it predominantly affects males, with females usually being asymptomatic carriers who may pass the mutation onto their children.
In the United States, the number of diagnosed cases is relatively low compared to other common genetic eye diseases. However, the incidence of choroideremia is expected to increase with greater awareness, improved genetic testing, and diagnostic advances. The disease is most commonly diagnosed in early childhood when a child begins to experience difficulty seeing in dim light and may show signs of night blindness.
Regions with higher awareness of genetic disorders and advanced healthcare systems (such as North America and Europe) are expected to see an increase in diagnoses, whereas emerging markets in Asia and Africa are less likely to diagnose the condition due to limited access to genetic testing and ophthalmic care.
Choroideremia Market Drivers
-
Advances in Gene Therapy: The development of gene therapies is the most significant driver in the choroideremia treatment market. Gene-based therapies such as voretigene neparvovec have shown potential in treating inherited retinal diseases by targeting the genetic mutations that cause the disease. The approval of similar gene therapies specifically for choroideremia is expected to transform treatment paradigms.
-
Increasing Diagnosis and Awareness: With the rise of genetic testing and improvements in diagnostic techniques, such as optical coherence tomography (OCT) and fundus autofluorescence imaging, there is an increase in the diagnosis of choroideremia. Public and healthcare awareness of inherited retinal diseases is also improving, leading to earlier identification of the condition, which drives demand for therapeutic interventions.
-
Collaborations and Investments in Rare Diseases: With choroideremia being a rare disease, pharmaceutical and biotechnology companies are forming strategic partnerships and investing in the development of innovative treatments. Collaborations between research institutions and pharmaceutical companies, along with government incentives such as Orphan Drug Designation and Rare Pediatric Disease Priority Review Voucher, are accelerating the development of treatments for choroideremia.
-
Government and Regulatory Support: Governments worldwide are increasingly funding research into rare diseases like choroideremia. Regulatory agencies, including the FDA and EMA, are offering fast-track approval pathways and additional incentives for developing therapies for rare, life-threatening conditions. This regulatory support is crucial for bringing potential therapies to market faster.
Request for sample report @ Choroideremia Market
Competitive Landscape
The choroideremia market is currently in a development phase, with few therapies approved and many still undergoing clinical trials. Some of the leading companies involved in the development of treatments for choroideremia include:
-
Spark Therapeutics: Spark Therapeutics is known for its pioneering work in gene therapy, particularly in Luxturna, a gene therapy for inherited retinal diseases. They are actively exploring gene-based therapies for choroideremia, and their advancements in retinal gene therapy are closely monitored.
-
Avalanche Biotech: Avalanche Biotech is developing gene therapies for retinal diseases, including choroideremia. Their efforts are focused on leveraging viral vector technology to deliver genes to the retina to restore visual function.
-
GenSight Biologics: GenSight is developing GS030, a gene therapy for retinal diseases, including choroideremia. Their approach involves a combination of gene therapy and optogenetic treatment, which aims to restore vision by using light to stimulate retinal cells.
-
EDIT-101 by Editas Medicine: Editas Medicine, a leader in CRISPR gene editing, is exploring the use of EDIT-101 for inherited retinal diseases, including choroideremia. CRISPR technology holds significant promise for treating genetic disorders at the DNA level.
-
Bayer and REGENXBIO: Bayer, in partnership with REGENXBIO, is advancing RGX-314, a gene therapy designed to treat retinal diseases, including choroideremia. Their research aims to develop a one-time treatment that can provide lasting effects.
Choroideremia Market Forecast - 2032
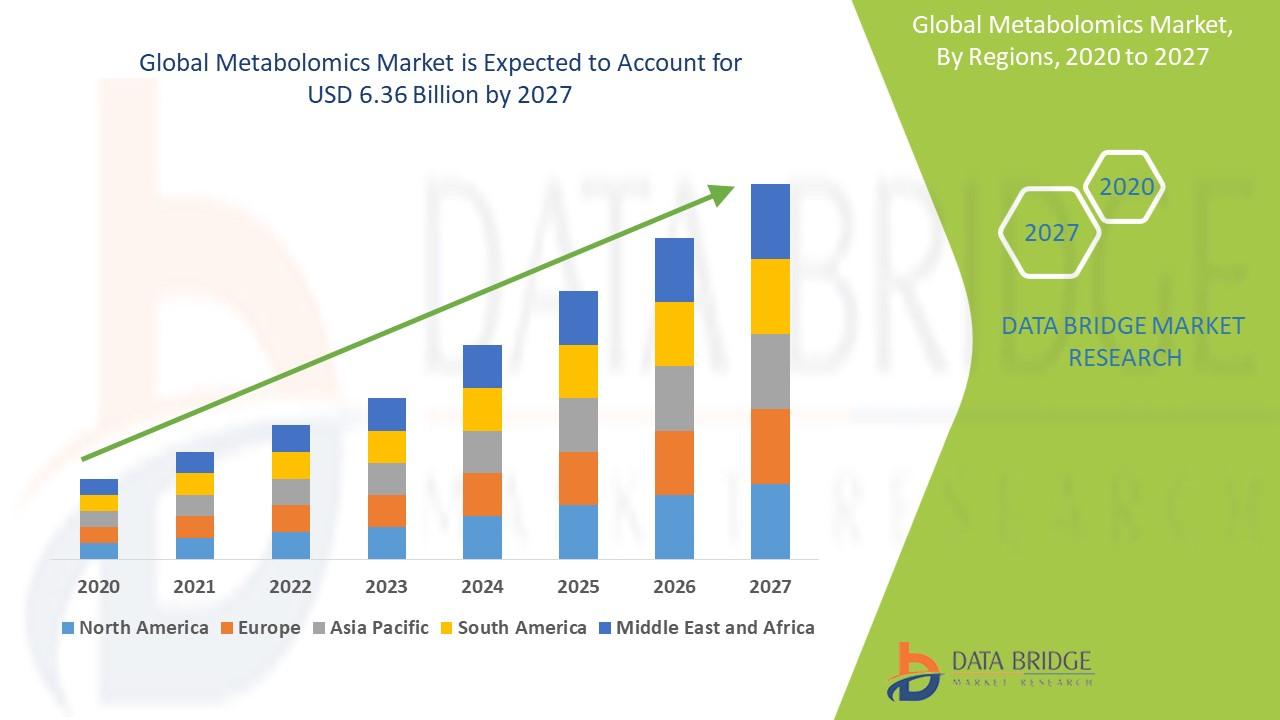
The choroideremia market is expected to grow significantly in the coming years, driven by the ongoing development of gene therapies, increasing diagnostic capabilities, and heightened awareness of the condition. By 2032, the market is expected to reach USD 1-2 billion, with growth fueled by the introduction of gene therapies and other advanced treatment modalities.
Key factors influencing the market include:
-
The launch of the first FDA-approved gene therapies specifically targeting choroideremia.
-
Growth in the number of clinical trials and partnerships in the retinal gene therapy space.
-
Rising demand for personalized medicine and genetically tailored therapies.
-
Expansion of genetic testing and diagnostic tools in both developed and emerging markets.
Request for sample report @ Choroideremia Market
Conclusion
Choroideremia remains a rare but serious eye disease with significant unmet medical need. The market for choroideremia treatments is evolving rapidly, driven by advancements in gene therapy, increased awareness, and the expansion of diagnostic capabilities. As the landscape for choroideremia treatment continues to evolve, there is considerable hope that gene therapies and other innovative treatments will improve patient outcomes and offer a new lease on life for individuals living with the condition. The market forecast for 2032 is positive, with substantial growth expected due to the continued progress in treatment development and increased support for rare disease research.
Latest Report Offered By DelveInsight:
Benefits Of Robotics In Healthcare | Lewy Body Dementia | Energy Based Aesthetic Devices Market | Ependymoma Market | Fertility Monitoring Devices Market | Germ Cell Tumor Market | Hernia Repair Devices Market | Hot Flashes Market | Implantable Cardioverter Defibrillators Market | Keloid Market | Orthopedic Power Devices Market | Pouchitis Market | Surgical Sealant Market | Transthyretin Amyloidosis Market | Vascular Graft Devices Market | Lip And Oral Cavity Cancer Market | Sinus Dilation Devices Market | Inguinal Hernia Market | Plaque Psoriasis Market | Plasmodium Vivax Malaria Market | Hdac Inhibitors Market | Peritoneal Dialysis Equipment Market | Adenosine Deaminase-severe Combined Immunodeficiency Market | Bone Resorption Market | Pelvic Inflammatory Disease Market